-
Company
Introduction -
Product
-
Production &
Quality -
Contact Us

- HOME
- Production & quality
- Quality
Production & Quality
Quality
BionPak is ceaselessly putting in efforts for continuous advancement of the quality control system by executing analysis of the products on the basis of the pharmacopoeia standards USP, EP and KP, etc.
Product analysis specifications
- Base weight - ASTM D3776
- Thickness - ASTM D1777
- Dimensions - JIS-L-1096
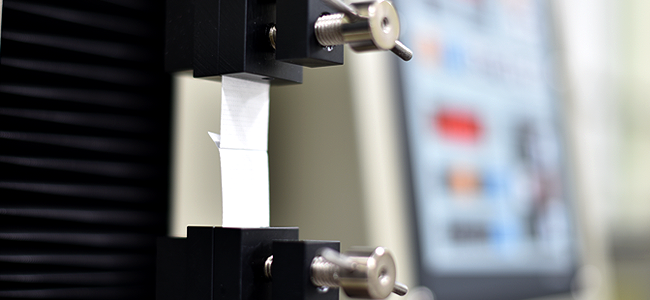
- Binding strength - ASTM F88
- Seal Leak - ASTM F1929
- BI Test - USP <1229>

Products quality specifications
- Cytotoxicity - ISO 10993-5
- Bioburden - ISO 11737-1
- Endotoxins - USP <85>
- Sterilization validity - ISO11737-2

Raw material quality specifications
- Latex Free
- RoHS
- BSE / TSE
- Reach Regulation
- OEKO TEX
- Food Contact
- Food Allergens
- SVHC(Substances of Very High Concern)
Quality service
- Execute user sealing and binding strength test (ASTM F88)
Clean room environment management
- ISO class 5 & 7 annual validation
- Execute real-time floating particle monitoring
Clean room analysis environment
- Clean bench (ISO 5) for quality test
- Manufacturing facility Cleanness grade (ISO 5 & 7)













